Nitrogen Energy Levels
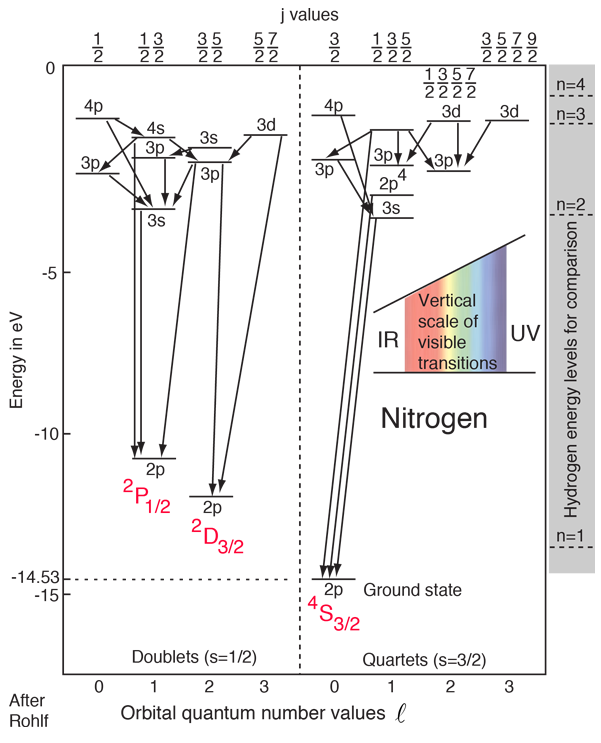
With an electron configuration of 1s22s22p3, the element nitrogen has three electrons outside closed shells. The three spins can give a resultant of spin 3/2 (quartet states) or 1/2 (doublet states). In the diagram above, it is presumed that two of the electrons remain in their lowest states, and the lower case label on the levels specifies the state of the elevated electron. The upper case labels represent the collective states of the three p electrons.
The ground state has all three spins aligned in the 4S3/2 state, the highest multiplicity state, consistent with Hund's rule #1. There are two other 2p states associated with spin S = 1/2 (doublets) for which the collective symbols are 2P1/2 and 2D3/2. The 2D3/2 state is lower in accordance with Hund's rule #2. The splitting with the different j values is not shown here.
The lines connecting levels indicate radiative transitions, which are subject to selection rules depending upon the angular momenta.
| Electron energy level diagrams |
Reference
Rohlf
Ch 9
| HyperPhysics***** Quantum Physics | R Nave |