Technetium-99m
Technetium-99m is a widely used radioactive tracer isotope in Nuclear Medicine. It's gamma ray energy of about 140 keV is convenient for detection. The fact that both its physical half-life and its biological half-life are very short leads to very fast clearing from the body after an imaging process. A further advantage is that the gamma is a single energy, not accompanied by beta emission, and that permits more precise alignment of imaging detectors.
| Isotope | |||
| 99mTc | |||
Technetium -99m is produced by bombarding molybdenum 98Mo with neutrons. The resultant 99Mo decays with a half-life of 66 hours to the metastable state of Tc . This process permits the production of 99mTc for medical purposes. Since 99Mo is a fission product of 235U fission, it can be separated from the other fission products and used to generate 99mTc. For medical purposes, the 99mTc is used in the form of pertechnate, TcO4-.
The technetium isotope 99mTc is unusual in that it has a half-life for gamma emission of 6.03 hours. This is extremely long for an electromagnetic decay - more typical is 10-16 seconds. With such a long half-life for the excited state leading to this decay, this state is called a metastable state, and that is the reason for the designation 99m. Some aspects of the complex decay of this radioisotope are shown in the diagram below. The dominant decay mode gives the useful gamma ray at 140.5 keV.
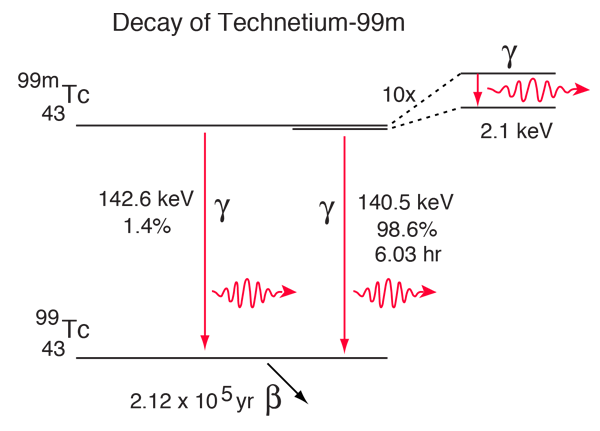
While the 140.5 keV gamma transition is labeled as happening 98.6% of the time, not all of those actually emit a gamma ray photon. The process called internal conversion always competes with gamma photon emission. This involves transfer of the energy of the transition to one of the atomic electrons, usually a K, L, or M shell electron. This decay has been studied carefully enough to know the fraction of the decays associated with each pathway.
|
In the K-internal conversion process, a K-shell electron is ejected with the transition energy 140.5 keV minus the binding energy of the K-shell electron. |
Nuclear applications to health
Reference
Hobbie
Ch. 16
| HyperPhysics***** Nuclear | R Nave |