Aspartic Acid
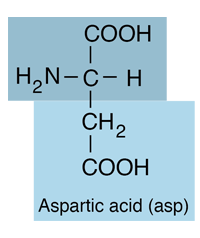 | Aspartic acid is an amino acid and belongs to the class which has acid or base R-groups. It has an acidic side chain and is hydrophilic. Under physiological conditions (pH 7.4) in proteins the side chain usually occurs as the negatively charged aspartate form, -COO-. |
Aspartic acid was first discovered in 1827 by Auguste-Arthur Plisson and Étienne Ossian Henry by hydrolysis of asparagine, which had been isolated from asparagus juice in 1806.
In the human body, aspartate is most frequently synthesized through the transamination of oxaloacetate. In plants and microorganisms, aspartate is the precursor to several amino acids, including four that are essential for humans: methionine, threonine, isoleucine, and lysine.
In the urea cycle, aspartate and ammonia donate amino groups leading to the formation of urea.
Aspartate participates in gluconeogenesis. It carries reducing equivalents in the malate-aspartate shuttle, which utilizes the ready interconversion of aspartate and oxaloacetate, which is the oxidized (dehydrogenated) derivative of malic acid. Aspartate donates one nitrogen atom in the biosynthesis of inosine, the precursor to the purine bases. In addition, aspartic acid acts as a hydrogen acceptor in a chain of ATP synthase. Dietary L-aspartic acid has been shown to act as an inhibitor of Beta-glucuronidase, which serves to regulate enterohepatic circulation of bilirubin and bile acids.
Aspartic acid is found in the sweetener aspartame.
D-Aspartate is one of two right handed acids commonly found in mammals.
| Aspartic acid wiki |
Biochemical concepts
Chemistry concepts
Reference
Tillery, Enger and Ross
Ch 14
Ahern
| HyperPhysics*****Chemistry *****Organic Chemistry | R Nave |