Cysteine
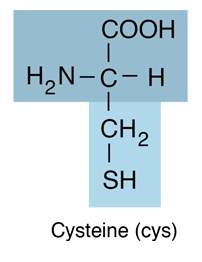 | Cysteine is an amino acid and belongs to the class which has neutral R-groups. It is hydrophilic. The thiol side chain in cysteine often participates in enzymatic reactions, as a nucleophile. The thiol is susceptible to oxidation to give the disulfide derivative cystine, which serves an important structural role in many proteins. |
Together with methionine, cysteine is one of two sulfur-containing proteinogenic amino acids.
Cysteine is an important source of sulfide in human metabolism. The sulfide in iron-sulfur clusters and in nitrogenase is extracted from cysteine, which is converted to alanine in the process.
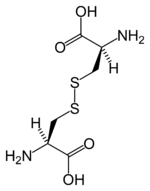 | Two cysteine molecules can bond to form cystine with a disulfide bond. In protein structures, disulfide bonds between cysteines from separate points in the polypeptide can influence the bending and shape of the protein. Insulin is an example of a protein with such cystine crosslinking. |
| Cysteine wiki |
Biochemical concepts
Chemistry concepts
Reference
Tillery, Enger and Ross
Ch 14
Ahern
| HyperPhysics*****Chemistry *****Organic Chemistry | R Nave |