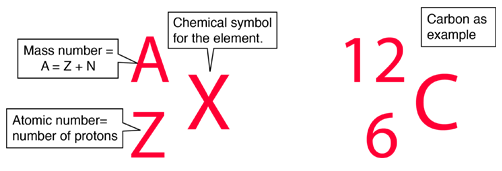Constituents of Atoms
The electrons, protons and neutrons which make up an atom have definite charges and masses. If they are modeled as hard spheres with the same density, they would have the relative sizes shown. While that model should not be taken as reality, it gives us a convenient object to which to attach the definite properties of the particles.
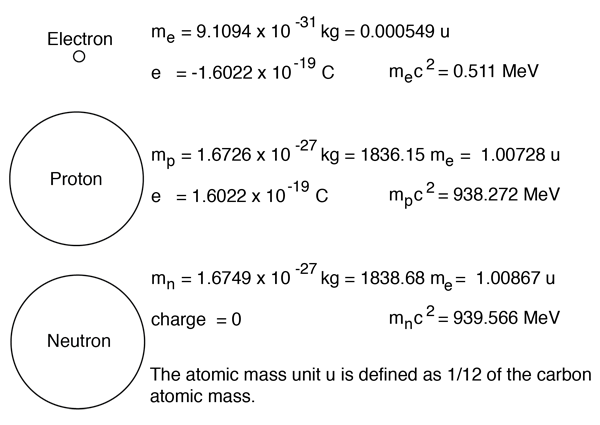
While the charges and masses are precisely known, the sizing is just fun and games. Our best information about the proton and neutron indicates that they are constituent particles, made up of three quarks each. They do however seem to have an effective density which is roughly characteristic of all nuclei, and we can attribute to them a radius of about 1.2 x 10-15 meters. The electron is a fundamental particle, classified as a lepton, which is apparently not made out of any constituent particles. Down to scales of a thousand times smaller than the proton radius quoted above, they have no apparent structure.
|
Index |