Visualizing Electron Orbitals
Rough sketches of the electron density for the first three shells of the hydrogen atom can give an impression of the constraints that govern the buildup of the periodic table. The limits on the occupation of the subshells arise from the quantum numbers for the atomic electrons and their relationship to each other. These sketches arise from the hydrogen wavefunctions which map the electron density.
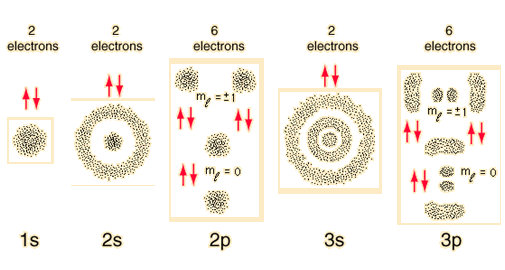
The numbers of the main shells, like 1s, arise from the principal quantum number n in the quantum mechanical description of the electrons. The letters designate the sub-shells and follow the historical spectroscopic notation. In general terms, the higher shells have higher energy (less tightly bound) and are on the average further out from the nucleus. This is strictly true for the hydrogen atom where the energy levels depend only upon the principal quantum number (fine structure neglected). But in larger atoms, the energy depends also upon the orbital quantum number so the sub-levels are filled in the order s, p, d, f, etc. This spreading eventually leads to overlap, with the 4s sub-level being lower in energy than the 3d sub-level.
The division into main shells encourages a kind of "planetary model" for the electrons, and while this is not at all accurate as a description of the electrons, it has a certain mnemonic value for keeping track of the buildup of heavier elements.
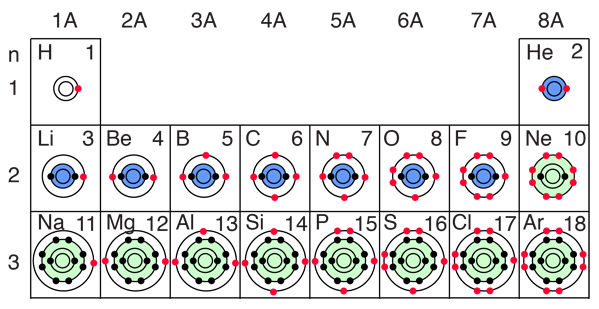
Perhaps a better way to keep track of the buildup process is to just track the filling of the states, emphasizing the order of buildup and the fact that the states are definite, quantized energy states. The Pauli exclusion principle tightly constrains the entire buildup process, allowing only one electron to occupy each available state. Since all of nature tends to the lowest energy state, the individual states will fill in the order of ascending energy.
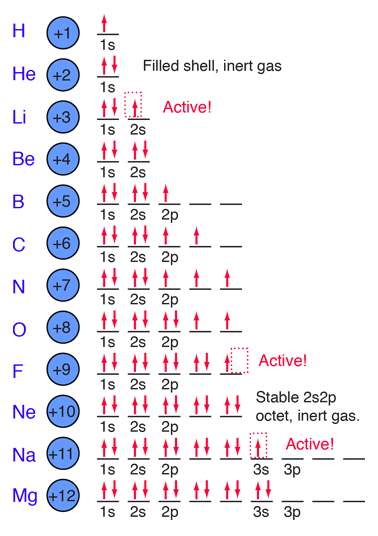 |
The buildup process for the first 12 elements of the periodic table gives some hints about the origin of chemical reactivity. The 1s spatial state can have two states of electron spin, which we usually just refer to as "spin up" and "spin down". With two electrons in the 1s state, it is filled and stable, forming the noble gas helium. It is chemically unreactive.
Lithium is a very active alkali metal, having just one electron in the second shell. It tends to lose that electron to revert to the stable helium electron structure. The elements from boron to neon in the periodic table are filling the 2p orbital. The three possibilities for 2p orbitals can be associated with spatial directions, say x,y and z. The order of filling will place one electron in each before placing two in any one orbital since the mutually repulsive electrons prefer to be further apart. |
The filling of the 2p orbital places eight electrons in the second shell, a "stable octet". The particularly stable configuration produces the noble gas neon. But fluorine with one less than a stable octet is very active! It tends to gain one electron while the very active sodium tends to lose one electron, in each case producing the stable octet configuration of neon.
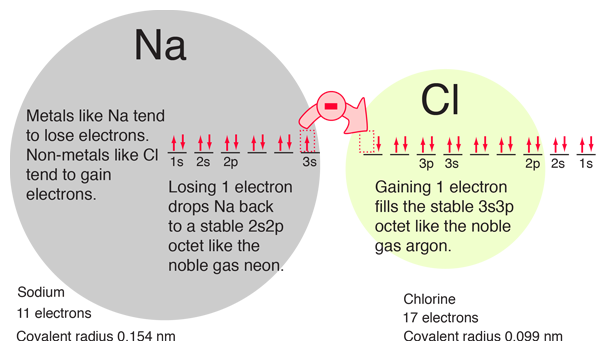
The electron orbital configurations provide a structure for understanding chemical reactions, which are guided by the principle of finding the lowest energy (most stable) configuration of electrons. We say that sodium has a valence of +1 since it tends to lose one electron, and chlorine has a valence of -1 since it has a tendency to gain one electron. Both of these atoms are very active chemically, and their combination is the classic case of an ionic bond.
The chemical compounds involving elements in the first three rows of the periodic table can be well understood with the kind of orbital considerations above. Things become more complicated in the fourth row of the periodic table. The 4s electrons dip down below the 3d electrons in energy, and since there are ten possible quantum number combinations in the d sub-shell, this inserts 10 elements between the 4s and 4p subshells, the so-called "transition elements".
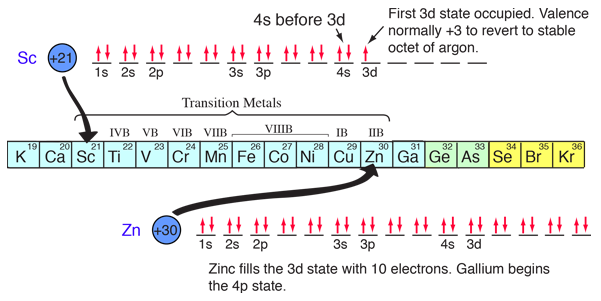
|
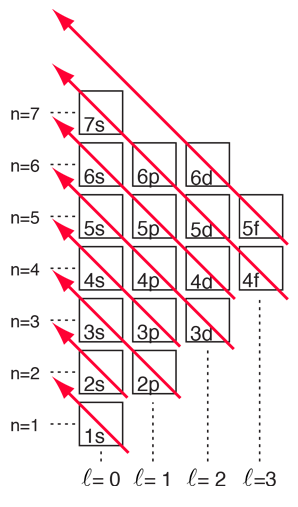
With the pattern established above, the order of filling of electron energy levels can be predicted in most cases. Although there are minor exceptions, the level crossing follows the scheme indicated in the diagram, with the arrows indicating the points at which one moves to the next shell rather than proceeding to higher orbital quantum number in the same shell. The electron configuration for any element may be found by clicking on that element in the periodic table. The first exception to the above scheme that is encountered is chromium, where the fifth 3d electron state is occupied instead of the second 4s state. Copper is also an exception, with all ten 3d levels being filled while the second 4s state is unoccupied. This exceptional behavior of chromium and copper is also exhibited by the elements just below them in the periodic table, molybdenum and silver. |
| Atomic properties | Ionization energies | Atomic radii |
| Periodic table of the elements | Element abundance, Earth's crust |
| HyperPhysics***** Chemistry | R Nave |