 |
Ionizing the AirVoltages of hundreds of thousands of volts can be generated with a demonstration model Van de Graaff generator. This is sufficient to ionize the air, which has primary constituents nitrogen and oxygen. An applied electric field will polarize air molecules, and if sufficient to force electrons off the molecules, those electrons can collide with other molecules and can start a discharge, or electric arc. The voltage necessary to start an arc depends upon the pressure and gap length, and is modeled in Paschen's law(Wikipedia). For long gaps in air at STP, the threshold for arc discharges is often quoted as 3.4 million volts per meter. When an atom is ionized, it will tend to collect a replacement electron which may cascade down though the available atomic energy levels, emitting light quanta characteristic of those level separations. The characteristic spectra of Nitrogen and Oxygen would be observed in a visible arc in air. |
A spherical metal shell is brought close to the van de Graaf generator, and a spark about 10cm long jumps to that sphere. The voltage required to produce an electric arc across a centimeter of air is in the neighborhood of 30,000 volts, so this arc may represent about 300,000 volts produced by the van de Graaf. | 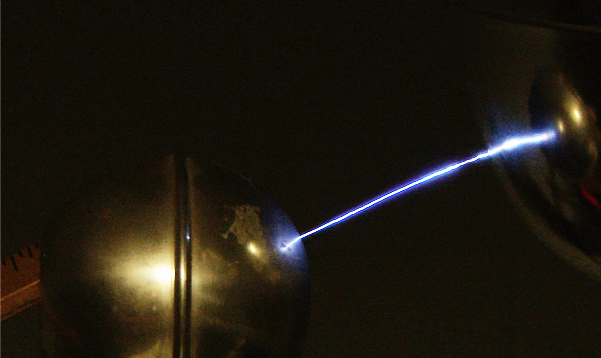 |
 | The van de Graaf can also be used to excite spectral tubes with specific gases in them and will display the characteristic colors for those gases. This gas is neon, and if the colors were separated by a diffraction grating, they would show the spectrum for neon gas. 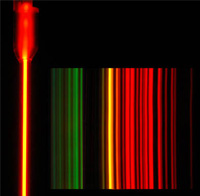 |
A cereal like puffed wheat or Cheerios provides a playful example of the mutual repulsion of objects that are given an electric charge of the same polarity. |  |
Voltage concepts
| HyperPhysics***** Electricity and Magnetism | J Nave, R Nave |