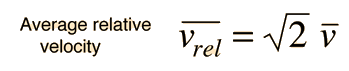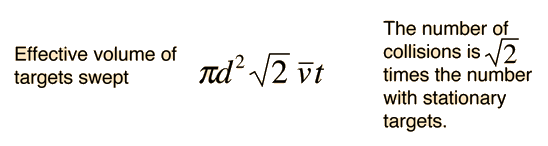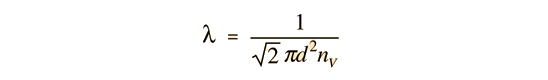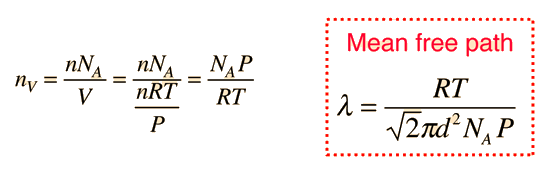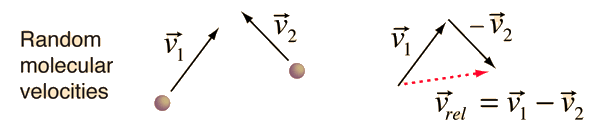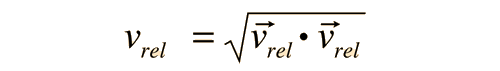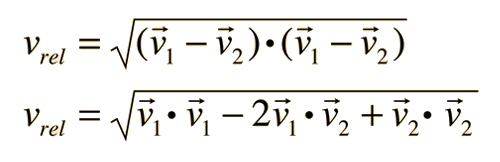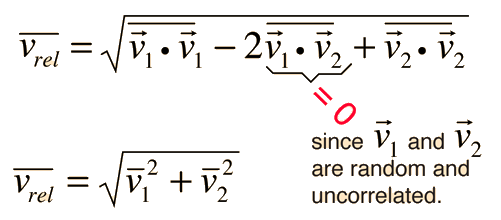Mean Free Path
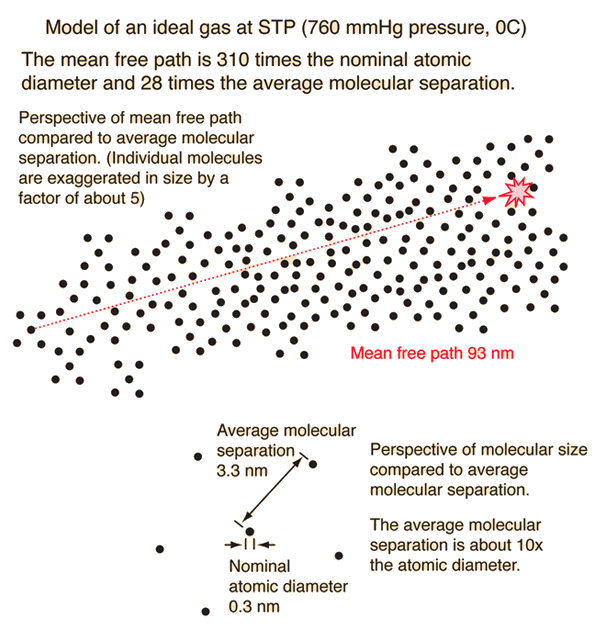
The mean free path or average distance between collisions for a gas molecule may be estimated from kinetic theory. Serway's approach is a good visualization - if the molecules have diameter d, then the effective cross-section for collision can be modeled by
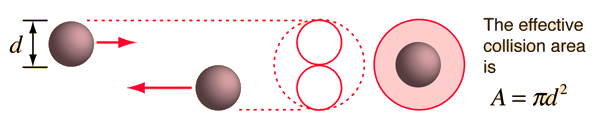
using a circle of diameter 2d to represent a molecule's effective collision area while treating the "target" molecules as point masses. In time t, the circle would sweep out the volume shown and the number of collisions can be estimated from the number of gas molecules that were in that volume.
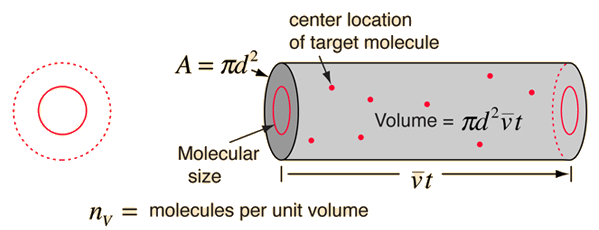
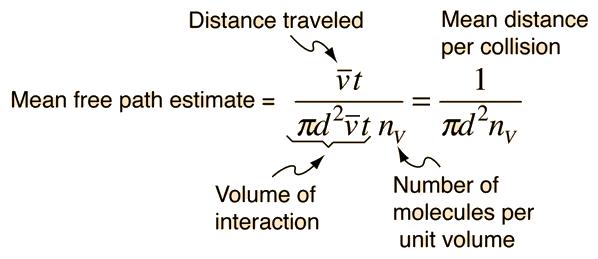
The mean free path could then be taken as the length of the path divided by the number of collisions.
The problem with this expression is that the average molecular velocity is used, but the target molecules are also moving. The frequency of collisions depends upon the average relative velocity of the randomly moving molecules.
| Refinement of mean free path | Calculation | Frequency of collision |
Gas law concepts
Kinetic theory concepts
Reference: Rohlf
Ch. 2
| HyperPhysics***** Thermodynamics | R Nave |