Pauli Repulsion in Ionic Molecules
An ionic bond may be modeled in terms of the ionization energy to produce the positive ion, the electron affinity associated with the negative ion, the dissociation energy for the molecule, the coulomb potential between the ions, and the repulsive force which limits the closeness of approach of the ions. This repulsive force is typically called Pauli repulsion. The energy balance of all these terms can be written in the form
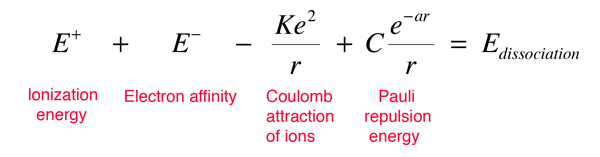
Measured data about ionic diatomic molecules allow us to imply the energy of the Pauli repulsive force at the equilibrium separation. It is modeled above with two parameters C and a which can be adjusted to fit the data. This repulsive force is more than just an electrostatic repulsion between the electron clouds of the two atoms. It has a quantum mechanical character rooted in the Pauli exclusion principle, and is often called just the "exclusion principle repulsion". When the ions are widely separated, the wavefunctions of their core electrons do not significantly overlap and they can have identical quantum numbers. As they get closer, the increasing overlap of the wavefunctions causes some to be forced into higher energy states. No two electrons can occupy the same state, so as a new set of energy states is formed for the composite, two-nucleus system, the lower energy states are filled and some of the electrons are pushed into higher states. This requires energy and is experienced as a repulsion, preventing the ions from coming any closer to each other. The nature of the Pauli repulsion term for sodium chloride is shown in the energy diagram below.
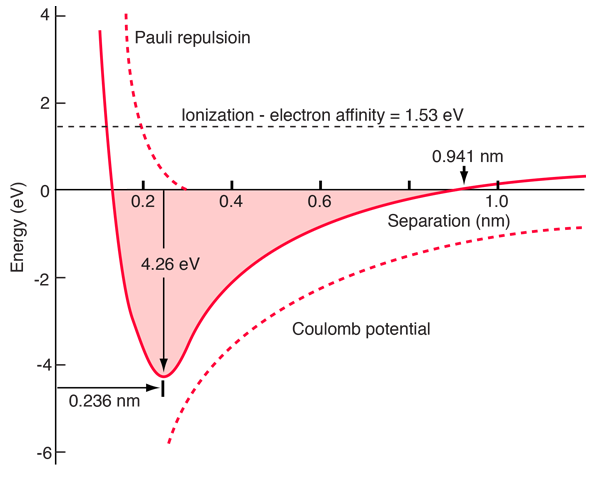
Since the ionization energies, electron affinities, and dissociation energies have been tabulated from experiment, and since the bond length is obtainable from independent experiments such as rotational spectroscopy, it is possible to estimate the energy of the Pauli repulsion by using the relationship above. Some examples are shown below, with the Pauli term calculated from the other data as the value which would balance the energy.
| Molecule | Positive Ion | Negative Ion | (Bond length) | at Equilibrium | for Energy Balance | |||||||
For the alkali halides, the Pauli term is a few tenths of an eV. The values for the Pauli term for the hydrides is enough different to suggest that something different is going on, but I lack the chemical insight to comment further on that. Any comments would be welcomed.
| Chemical bonds |
Reference
Rohlf
Sec 10.2
| HyperPhysics***** Quantum Physics ***** Chemistry | R Nave |