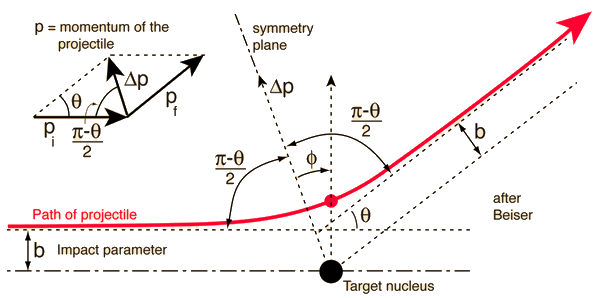
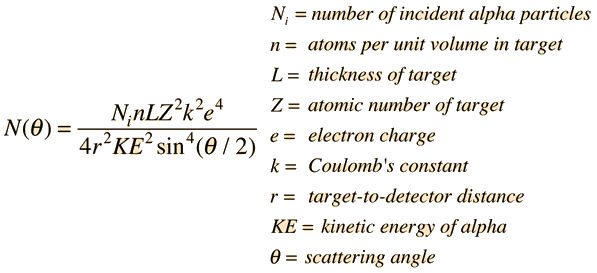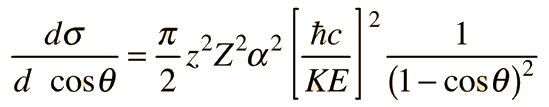Rutherford Scattering
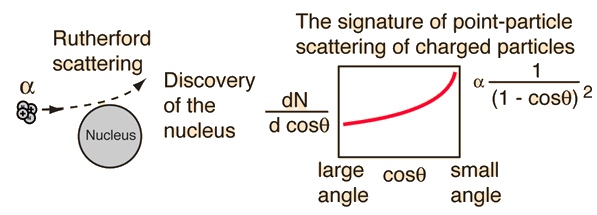
Alpha particles from a radioactive source were allowed to strike a thin gold foil. Alpha particles produce a tiny, but visible flash of light when they strike a fluorescent screen. Surprisingly, alpha particles were found at large deflection angles and some were even found to be back-scattered.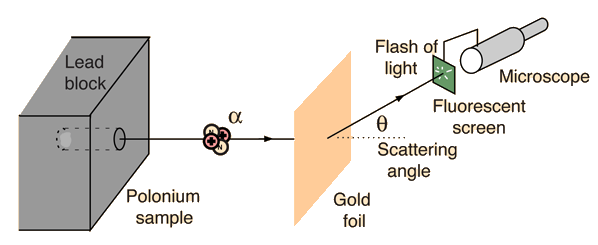
This experiment showed that the positive matter in atoms was concentrated in an incredibly small volume and gave birth to the idea of the nuclear atom. In so doing, it represented one of the great turning points in our understanding of nature.
If the gold foil were 1 micrometer thick, then using the diameter of the gold atom from the periodic table suggests that the foil is about 2800 atoms thick.
| Some history | Questions raised | Geiger-Marsden data |
Rutherford concepts
Scattering concepts
Great experiments of physics
| HyperPhysics***** Mechanics ***** Nuclear | R Nave |