Water & Ice, Water & Steam
How can you have water and ice at the same temperature? How can you have water and steam at the same temperature?
The key idea is that temperature does not measure the entire internal energy of a substance, only the translational kinetic energy part. During a phase change, the energy goes into the potential energy part, either taking from or adding to the energy associated with the molecular attractions.
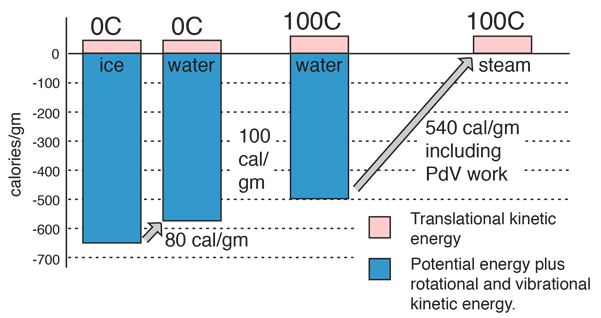
The constancy of temperature when water is going through its phase changes at 0°C and 100°C provides some insight into the nature of the internal energy of water, ice, and steam and into the nature of temperature. The large size of the phase change energy indicates that there is a large amount of potential energy associated with the intermolecular forces between the water molecules. During the melting and vaporization phase changes, the energy goes into the breaking down of the solid or liquid bonds, reducing the binding potential energy by the amount of the phase change energy and therefore not changing the average translational kinetic energy as reflected by the temperature.
| Heat of fusion | Division of energy | Heat of vaporization |
| Heat Questions |
Heat concepts
| HyperPhysics***** Thermodynamics | R Nave |