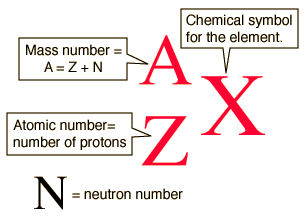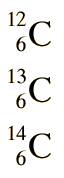Isotopes
The different isotopes of a given element have the same atomic number but different mass numbers since they have different numbers of neutrons. The chemical properties of the different isotopes of an element are identical, but they will often have great differences in nuclear stability. For stable isotopes of light elements, the number of neutrons will be almost equal to the number of protons, but a growing neutron excess is characteristic of stable heavy elements. The element tin (Sn) has the most stable isotopes with 10, the average being about 2.6 stable isotopes per element.

Information about the isotopes of each element and their abundances can be found by going to the periodic table and choosing an element. Then take the link to nuclear data.
Isotopes are (almost) Chemically Identical
It is significant to note that the three isotopes of hydrogen change in mass by a factor of three, but their chemical properties are virtually identical. A tiny difference in the spectral frequencies of hydrogen and deuterium comes from an essentially mechanical source, the slight change in the "reduced mass" associated with the orbiting electron. But for practical purposes the chemical behavior of the isotopes of any element are identical.
The dominant contributer to the interactions between atoms and their environment is the electromagnetic force. It should not be surprising that an extra neutron or two in the nucleus has almost no effect on that interaction with the world. Examination of a scale model of the atom makes it evident that the nucleus is extremely tiny compared ot the size of the atom. The nuclear radius of carbon-12 is 2.7 x 10-15 m while the size of the atom from the periodic table is about 0.9 x 10-10 m, about 33,000 times larger!
|